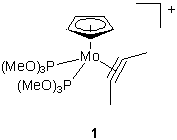
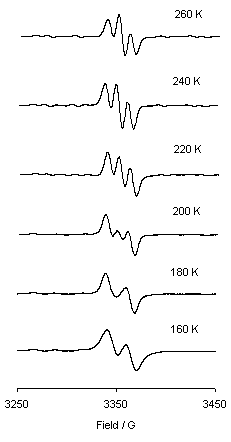
Working in collaboration with Professor Connelly and his group, I've been looking at the ESR spectra of some 19- and 17- electron alkyne compounds of (mainly) molybdenum. Ions such as 1 (right) have a relativey low-lying LUMO, and can be chemically reduced by decamethylcobaltocene. The resulting radicals are not stable enough to be isolated, but are readily detected by ESR, and show some interesting properties. At high temperatures the alkyne moves back and forth in a windscreen-wiper fashion, meaning that the two phosphorus atoms are in the same environment, and the resulting ESR pattern is a triplet. As the temperature is lowered though, the motion of the alkyne is slowed until it becomes essentially stationary. At this point it lines up nearer to one phosphorus atom than the other, rendering them inequivalent, and the pattern changes to a doublet. The resonance of an electron is so fast (GHz, compared to MHz for protons in NMR) that for a compound to be fluxional on the ESR timescale is unusual; for this to be temperature dependant is exceedingly rare. See J. Chem. Soc., Chem. Commun., 2001, 2458, for further information.
We're currently doing some experiments with 13C labelled alkynes, in order to better see coupling to the central carbon atoms of the alkyne, which will (hopefully!) allow us to determine the unpaired electron density on these atoms, and thus their likely chemical behaviour.