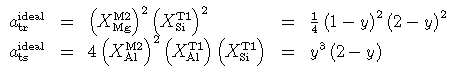
The reversed experimental determination of Al content of aluminous tremolites (Jenkins, 1994) provides powerful constraints on the mixing properties of amphiboles in this system. Jenkins compared several ideal mixing models with his reversals and noted that random mixing on both octahedral and tetrahedral sites was not supported by his experiments, and that a much more ordered situation was implied. Several such models, including those used by Jenkins, have been fitted to his experiments and the one which best fits the data is a partial-order model outlined below. Although the coupled one-site and two-site models of Jenkins yield close fits to his quartz-saturated experiments, they did not fit the silica-undersaturated data so well (Jenkins, 1994). There is also a logical inconsistency in using such models because they imply an ordered tschermakite end-member, yet the fits to the experiments demand a tschermakite entropy which is 40-50 J/K higher than estimates for ordered tschermakite. To resolve this, the new partial-order model assumes that Al and Mg mix on the M2 octahedral sites, but that there is less than maximal configurational entropy on the tetrahedral sites. Restricting the mixing of Al and Si to just the 4 T1 sites still yields too high an entropy to account satisfactorily for Jenkins' reversals, as he noted. Thus it will be assumed that the entropy contribution is half that for random mixing on the T1 sites, a situation which can be considered to correspond to charge-balanced coupling between an Al on M2 and one or other of the two nearest T1 site Al cations, rather than strict charge balance coupling to just one single tetrahedral neighbour. The activity of tremolite and tschermakite end-members in this model are just those of the random mixing model but with the square root of the tetrahedral site terms.
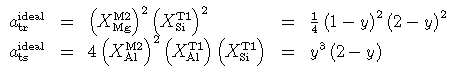
where y = XAlM2 is the mole fraction, or proportion, of the tschermakite end-member. The experimental data of Jenkins (1994) and Hoschek (1995) were used to determine the non-ideal mixing enthalpy, using a simple regular solution model
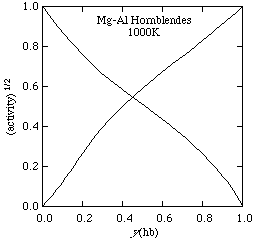
The square root of the activities at 1000K are shown below, with a value for Wtr-ts = 20 kJ/mol.
Coded up for THERMOCALC:
%========= Tremolite-tschermakite amphiboles in CMASH ===============
amp 2
y(am) 0.05
%-------------------------------------------------------------------
p(tr) 1 1 1 1 -1 y
p(ts) 1 1 0 1 1 y
% --------------------------------------------------
sf
W(trts) 20 0 0
% --------------------------------------------------
4 % "site fractions"
xAlM2 1 1 0 1 1 y
xMgM2 1 1 1 1 -1 y
xAlT1 1 1 0 1 1/2 y
xSiT1 1 1 1 1 -1/2 y
% --------------------------------------------------
% ideal mixing activities: may only involve "site fractions"
tr 1 2 xMgM2 2 xSiT1 2
ts 4 3 xAlM2 2 xAlT1 1 xSiT1 1
% __________________________________________________________________
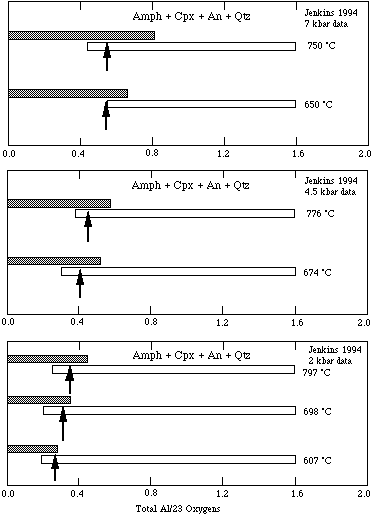
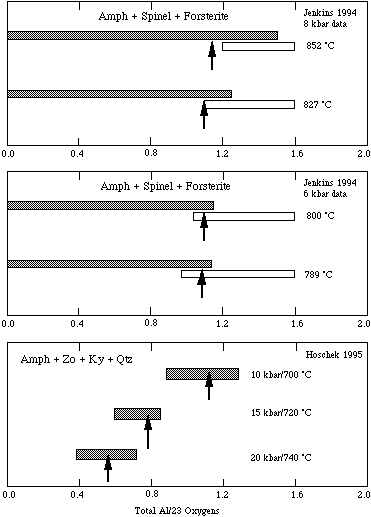
The experimental reversals of Jenkins (1994) involving both silica-saturated and -undersaturated assemblages as well as those of Hoschek (1995) are well fitted, as may be seen in the figures below, with this reduced-entropy mixing model. The derived enthalpy of the tschermakite end-member depends on this mixing model.
|
|