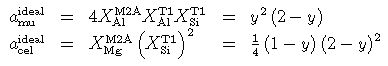
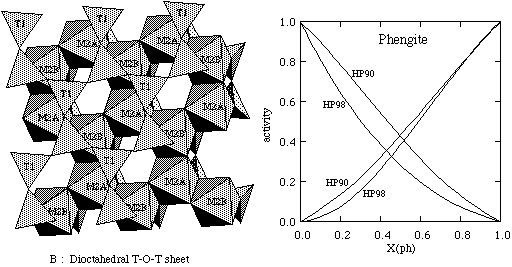
KMASH: The ideal mixing model used in HP90 involved mixing of octahedral Al and Mg on the two M2 sites in the dioctahedral mica structure, M1 being vacant. This may be unrealistic given that Mg-Al ordering is such a strong control on the behaviour of octahedral site distributions (e.g. in omphacite and chlorite). Thus it is anticipated that each Mg entering the dioctahedral sheet will tend to be placed such that its nearest neighbours are all Al. This short range order situation is not easy to model satisfactorily, but energetically this would be much more like a hypothetical fully ordered mica in which Al and Mg can only mix on one (M2A) of the two sites, such that each M2A is surrounded by three M2B sites and vice versa as shown below.
The mixing of tetrahedral Al and Si is restricted to just two of the four T sites to maintain Al-avoidance, as also argued in HP90.
|
|
|
|
|
|
|
|
|
|
|
|
Thus the activity model for KMASH muscovite-celadonite micas is
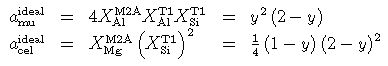
And, coded for THERMOCALC:
% ============= Muscovite-celadonite ========================
mu 2
y(mu) 0.95
p(cel) 1 1 1 1 -1 y
p(mu) 1 1 0 1 1 y
sf
W(cel,mu) 0 0 0
4 % 4 site fraction terms
X(Mg,M2a) 1 1 1 1 -1 y
X(Al,M2a) 1 1 0 1 1 y
X(Si,T1) 1 1 1 1 -1/2 y
X(Al,T1) 1 1 0 1 1/2 y
cel 1 2 X(Mg,M2a) 1 X(Si,T1) 2
mu 4 3 X(Al,M2a) 1 X(Al,T1) 1 X(Si,T1) 1
%__________________________________________________________________
where y (the fraction of Al in M2A) is the mole fraction, or proportion, of muscovite in the white mica. Fitting the Si contents of phengites in the experiments of Massonne & Schreyer (1987, 1989) with a non-ideal regular solution model yielded values for W(mu-cel)=0 within error, and so phengitic micas are taken to be approximately ideal for the above mixing activities. The derived enthalpy of celadonite depends upon this mixing model, whereas that of the muscovite end-member is independent of any mixing model. The activities differ from those of HP90, and care should be taken to use the new model when calculating with the new dataset.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
and a second compositional parameter x = Fe/(Fe+Mg). In the case of ideal mixing the activities become:
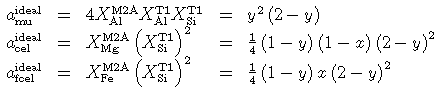
And, coded for THERMOCALC:
% ============= Fe-Mg Muscovites ========================
mu 3
x(mu) 0.84
y(mu) 0.81
% --------------------------------------------------
p(mu) 1 1 0 1 1 y
p(cel) 1 2 1 1 -1 x 1 1 -1 y
p(fcel) 1 2 0 1 1 x 1 1 -1 y
% --------------------------------------------------
ideal
% --------------------------------------------------
5 x(Al,M2A) 1 1 0 1 1 y
x(Mg,M2A) 1 2 1 1 -1 x 1 1 -1 y
x(Fe,M2A) 1 2 0 1 1 x 1 1 -1 y
x(Al,T1) 1 1 0 1 1/2 y
x(Si,T1) 1 1 1 1 -1/2 y
% --------------------------------------------------
mu 4 3 x(Al,M2A) 1 x(Al,T1) 1 x(Si,T1) 1
cel 1 2 x(Mg,M2A) 1 x(Si,T1) 2
fcel 1 2 x(Fe,M2A) 1 x(Si,T1) 2
NKFMASH: Addition of Na to the mica system leads to non-ideal
mixing of Na-K on the large A site, and the generation of an
asymmetric solvus. This can conveniently be dealt with by a regular
solution of the K end-members with a fictive paragonite end-member.
The parameters are fitted to the empirical mu-pa solvus of Eugster et
al. (J Pet 1972) and Guidotti et al. (J Met Geol 1994).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The parameter z = Na/(Na+K) describes the introduction of Na into the ideal activity model:
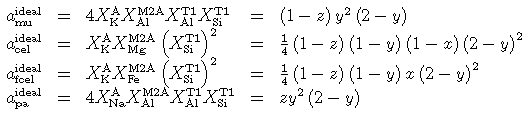
and the non-ideal part is given by a regular solution model (the proportions of end-members can be substituted as pa = z, cel = (1 - x )(1 - y ), fcel = x (1 - y ), mu = y - z ):
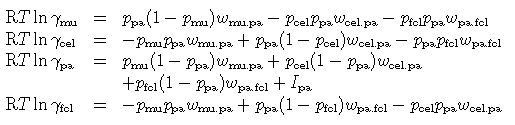
where the regular solution parameter W and the DQF Gibbs energy increment for the fictive paragonite end-member I pa have been derived from the muscovite-paragonite solvus of Eugster et al. (J Pet 1972). The other values are assigned so as to fit the empirical solvus as a function of P-T-X by Guidotti et al. (J Met Geol 1994):
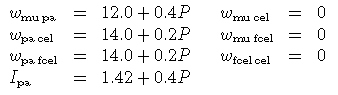
And, coded up for THERMOCALC:
% =========== NKFMASH muscovites ====================
mu 4
x(mu) 0.78 % bulk Fe/(Fe + Mg)
y(mu) 0.97 % x(Al,M2)
z(mu) 0.09 % x(Na,A)
% --------------------------------------------------
p(mu) 1 1 0 2 1 y -1 z
p(pa) 1 1 0 1 1 z
p(cel) 1 2 1 1 -1 x 1 1 -1 y
p(fcel) 1 2 0 1 1 x 1 1 -1 y
% --------------------------------------------------
sf
W(mu,pa) 12 0 0.4
W(mu,cel) 0 0 0
W(mu,fcel) 0 0 0
W(pa,cel) 14 0 0.2
W(pa,fcel) 14 0 0.2
W(cel,fcel) 0 0 0
% --------------------------------------------------
7
x(Na,A) 1 1 0 1 1 z
x(K,A) 1 1 1 1 -1 z
x(Al,M2A) 1 1 0 1 1 y
x(Mg,M2A) 1 2 1 1 -1 x 1 1 -1 y
x(Fe,M2A) 1 2 0 1 1 x 1 1 -1 y
x(Al,T1) 1 1 0 1 1/2 y
x(Si,T1) 1 1 1 1 -1/2 y
% --------------------------------------------------
mu 4 4 x(K,A) 1 x(Al,M2A) 1 x(Al,T1) 1 x(Si,T1) 1
pa 4 4 x(Na,A) 1 x(Al,M2A) 1 x(Al,T1) 1 x(Si,T1) 1
DQF 1.42 0 0.4
cel 1 3 x(K,A) 1 x(Mg,M2A) 1 x(Si,T1) 2
fcel 1 3 x(K,A) 1 x(Fe,M2A) 1 x(Si,T1) 2
|
|