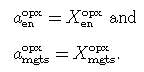
For En-Mgts orthopyroxenes, the simplest model is chosen, that of assumed charge coupling, whereby the tetrahedral site distribution is fixed by the occupancy of the nearest octahedral site, which leads to the simple relation in the MAS system
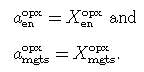
For En-Fs-Mgts orthopyroxenes, the symmetric formalism model of Holland & Powell (1996) and Powell & Holland (1998) may be used. It involves an ordered endmember fm, whose proportion is a function of composition and temperature; the proportion of fm, and hence the degree of order, is determined by THERMOCALC along with the other end-member proportions.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Again, tetrahedral terms are omitted (although in future some sort of order-disorder model involving tetrahedral sites will need to be addressed). Here, choosing the composition variables to be
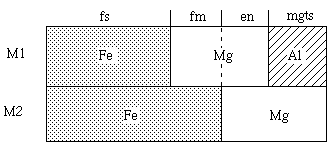
Then the site distributions become, in terms of these variables and in terms of the proportions of three independent end-members (mgts, en, fs)
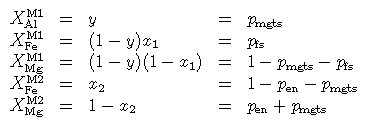
and the ideal mixing activities become
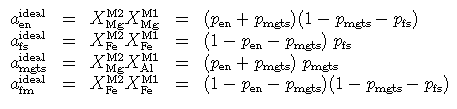
The activity coefficients are given by the regular solution model (mgts has been shortened to mt for clarity in the following):
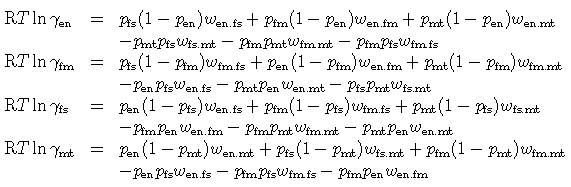
The values for the interaction energies are taken from Powell & Holland (1998 Am Min submitted):
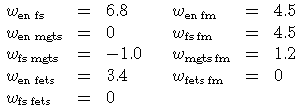
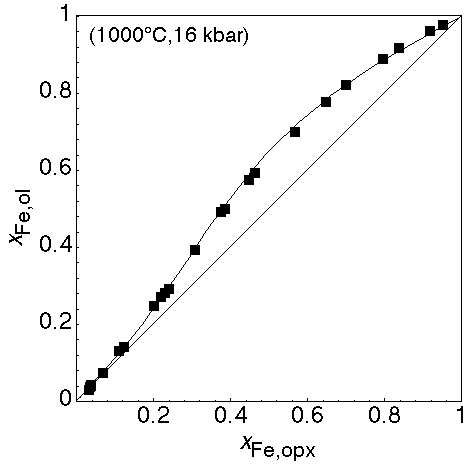
The data above perform quite well in fitting the experimental partitioning of Fe and Mg between olivine and opx (all curves in the following 3 figures were calculated using THERMOCALC):
as well as the Fe/Mg partitioning between garnet and orthopyroxene:
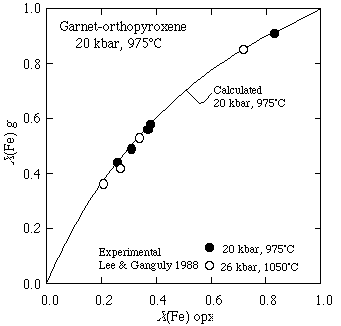
and the Al-solubility in orthopyroxene coexisting with garnet:
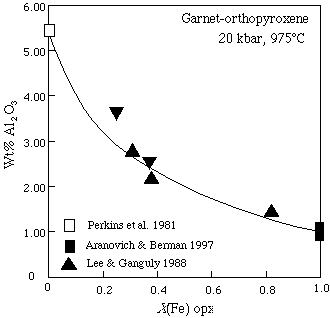
The interaction energies for the FAS system must include the dependent endmember fets (the Fe equivalent of mgts). The Gibbs energy of fets depends on the Gibbs energies of mgts, en and fm as well as the interaction energies:
The Gibbs energy of the fets end-member is constructed by THERMOCALC by supplying this information in the datafile.
Coded up for THERMOCALC the above becomes:
%========= Opx in FMAS ===============
% no tetrahedral mixing terms model
opx 4
x(opx) 0.2 % Fe/(Fe+Mg)
y(opx) 0.15 % x(Al,M1)
Q(opx) 0.15 % 2(x(Fe,M2) - x)
p(en) 1 1 1 3 -1 x -1 y -1/2 Q
p(fs) 2 1 0 1 -1/2 Q
2 0 1 1 x 1 1 -1 y
p(mgts) 1 1 0 1 1 y
p(fm) 2 1 0 1 1 Q
2 0 1 1 x 0 1 1 y
sf
W(en,fs) 6.8 0 0
W(en,mgts) 0 0 0
W(en,fm) 4.5 0 0
W(fs,mgts) -1 0 0
W(fs,fm) 4.5 0 0
W(mgts,fm) 1.2 0 0
5 x(Al,M1) 1 1 0 1 1 y
x(Mg,M1) 2 1 1 2 -1 y 1/2 Q
2 0 1 -1 x 1 1 -1 y
x(Fe,M1) 2 1 0 1 -1/2 Q
2 0 1 1 x 1 1 -1 y
x(Mg,M2) 1 1 1 2 -1 x -1/2 Q
x(Fe,M2) 1 1 0 2 1 x 1/2 Q
en 1 2 x(Mg,M1) 1 x(Mg,M2) 1
check 0 0 0
fs 1 2 x(Fe,M1) 1 x(Fe,M2) 1
check 1 0 0
mgts 1 2 x(Al,M1) 1 x(Mg,M2) 1
check 0 1 0
fm 1 2 x(Mg,M1) 1 x(Fe,M2) 1
check 1/2 0 1
make 2 en 1/2 fs 1/2
DQF -6.95 0 0
%__________________________________________________________________
|
|