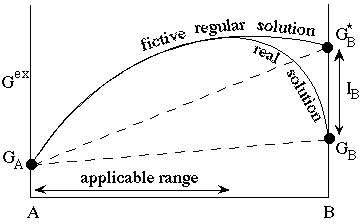
DQF (Darken's Quadratic Formalism). This simply states that over small (or sometimes quite large) ranges in composition, a complex solid solution can be approximated by a very simple model such as the regular solution model. Thus if we are interested in only one portion of a phase's composition space, such as one limb of a solvus, a regular model will suffice. In the excess Gibbs energy diagram shown below, a severely asymmetric solution may be modelled over a considerable range in composition by assuming a symmetric (regular) solution which extends from the real A with Gibbs energy GA to a fictive endmember B with Gibbs energy GB*.

The DQF parameter IB represents the increment to the Gibbs energy of phase B in the model. In the range of applicability shown the activity coefficients are given simply by
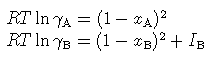
Perhaps the most obvious and natural place to use the DQF approach is where there is a structural phase transition in a solid solution which breaks it naturally into two or more regions. A good example is the plagioclase feldspar solid solution, where the structure is Cbar1 for compositions below around X an =0.5 and Ibar1 for more anorthitic compositions (depending on temperature):
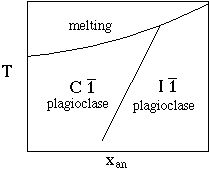
A simple regular solution in each region, with one real and one fictive endmember, appears to work quite well. In the C1 region the solid solution is described in terms of a real albite end-member which mixes with a fictive C1 anorthite end-member, and in the I1 region a real anorthite mixes with a fictive I1 albite.
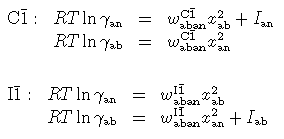
where
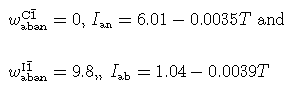
(Holland & Powell (Am Min 1992); all units in kJ/mol). The calculated activities at 700°C are as follows (almost identical to those of Orville 1972).
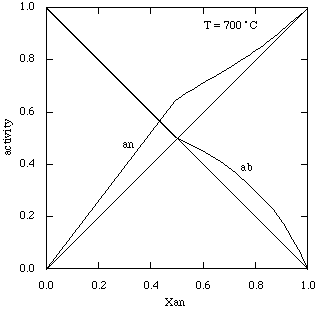
Coded up for THERMOCALC:
% ============= DQF Cbar1 plagioclase ====================
Cpl 2
Ca(pl) 0.05
%-------------------------------------------------------------------
p(an) 1 1 0 1 1 Ca
p(ab) 1 1 1 1 -1 Ca
% --------------------------------------------------
sf
W(aban) 0 0 0
% --------------------------------------------------
2 % 2 "site fractions"
xCaA 1 1 0 1 1 Ca
xNaA 1 1 1 1 -1 Ca
% --------------------------------------------------
% ideal mixing activities
an 1 1 xCaA 1
DQF 6.01 -0.0035 0
ab 1 1 xNaA 1
%__________________________________________________________________
% ============= DQF Ibar1 plagioclase ====================
Ipl 2
Ca(pl) 0.8
%-------------------------------------------------------------------
p(an) 1 1 0 1 1 Ca
p(ab) 1 1 1 1 -1 Ca
% --------------------------------------------------
sf
W(aban) 9.8 0 0
% --------------------------------------------------
2 % 2 "site fractions"
xCaA 1 1 0 1 1 Ca
xNaA 1 1 1 1 -1 Ca
% --------------------------------------------------
% ideal mixing activities
an 1 1 xCaA 1
ab 1 1 xNaA 1
DQF 1.38 -0.0039 0
%__________________________________________________________________
|
|