MASH: The activity model for talc is the same as used in HP90. Talc having a similar structure to that of mica discussed above, the Al is assumed to order onto one of the octahedral sites (M3), and to enter only the two T2 sites.

The full FMASH activities are given below, by the expressions used in HP90 (the diagram above is for MASH, setting x to zero below):
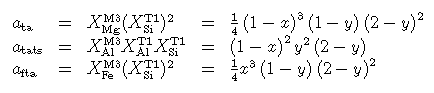
where y (= X Al,M3) is the mole fraction, or proportion, of tats in the talc, and x is Fe/(Fe+Mg). While the derived enthalpy of aluminous talc (tats) depends on this mixing model, that of talc does not.
Coded example for THERMOCALC:
%============== FeMgAl Talc =====================
ta 3
y(ta) 0.005
x(ta) 0.05
% --------------------------------------------------
p(ta) 2 1 0 1 -2/3 y
2 1 1 -1 x 1 1 -1/3 y
p(tats) 1 1 0 1 1 y
p(fta) 1 2 0 1 1 x 1 1 -1/3 y
% --------------------------------------------------
sf
W(ta,tats) 0 0 0
W(ta,fta) 0 0 0
W(tats,fta) 0 0 0
% --------------------------------------------------
7 % 7 site fraction terms.
xAlm3 1 1 0 1 1 y
xMgm3 1 2 1 1 -1 x 1 1 -1 y
xFem3 1 2 0 1 1 x 1 1 -1 y
xMgm1 1 1 1 1 -1 x
xFem1 1 1 0 1 1 x
xAlt1 1 1 0 1 1/2 y
xSit1 1 1 1 1 -1/2 y
% --------------------------------------------------
% ideal mixing activities in terms of "site fractions"
ta 1 3 xMgm3 1 xMgm1 2 xSit1 2
tats 4 4 xAlm3 1 xMgm1 2 xSit1 1 xAlt1 1
fta 1 3 xFem3 1 xFem1 2 xSit1 2
% --------------------------------------------------
|
|