Research Overview
Crystal engineering
Since I came to Nottingham, I've been investigating multimodal and other multidentate ligands. The concept of multimodal ligands was formulated by N. R. Champness in the year 2000.1,2 Since that time many publications concerning multimodal have been published by Champness and other research groups. However it has been found that even if the ligands form really interesting structures, the probability to predict resulting structure is very low.
Molecular containers3
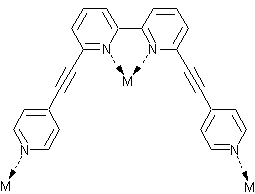
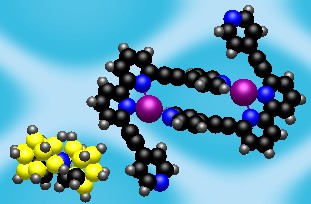
It has been shown before that multimodal ligands2 can be used for synthesis of peculiar coordination networks. The application of these ligands towards molecular containers is the story of my research. Thus, I started my work with the synthesis of multimodal ligands and the investigation of their coordination properties. We have designed the QPYE ligand (see the picture below) to produce discrete hexanuclear species by a reaction with tetrahedral metal centres.3 However the templating effect have been found to have a strong influence on the resulting structure. Thus we found that the reaction with AgX (X=BF4-, SbF6-) and CuBF4*4MeCN leads to hexanuclear cages, whereas in the case of AgNO3 or very large cobaltocarborate ([C2B9H11]2Co-) the reaction gives only binuclear species. The hexanuclear compounds were investigated using single crystal X-ray studies. It was found that in solid state one of the counteranions is accommodated within the cage. An interesting observation was made in the case of AgSbF6: the cage has lost its original (with BF4-) symmetry and the structure was solved in P-1 space group. The lost of the symmetry happens due to the necessity to accommodate large counteranion inside and to do that arms of two ligands are twisted in respect to corresponding bipyridyl fragments (see in the figure below). Further investigation of the compounds in solution were performed using NMR and ESI Mass spec. The stability of the Cu(I) cage in solution was shown by 19F NMR (BF4- and PF6- counteranions) and by mass spec. Instability of the Ag(I) cages has been shown using the same techniques.
 |
 |
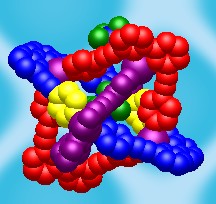 |
| View of the QPYE ligand and its binding modes | View of binuclear species [L2M2]2+ | View of hexanuclear species [L6M6(X)]5+ species. This structure corresponds to L6Ag6(SbF6)]5+. The two fragments are twisted to accommodate the counteranion and highlighted in yellow. |
See also: A movie illustrating the cage; a movie showing π-π interactions between cages in solid state.
The MolDraw Applet illustrating cages.
Extended structures: zeolite-like networks4
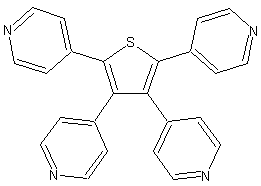
The coordination chemistry of the tetradentate thiophene based ligand TPT is another successful story of my research. Reactions of TPT with Ag(I) and Cu(I) salts leads to the formation of extended structures. Complexes formed by AgX (X=BF4-, SbF6-, PF6- and TfO-) and CuBF4 are isostructural and possess a zeolite-like framework with large pores. Thus the network consists of big (10x14Å), medium (18x7Å) octagonal and small (8x5Å) tetragonal pores.
 |
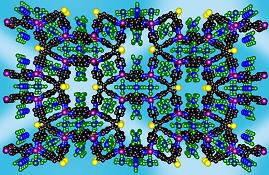 |
 |
| View of the TPT ligand | View of the overall framework. The pores are filled with the counteranions. | View of the topological framework. The silver node is shown in purple, ligand node - in black. |
The construction of topological network and topological
analysis of the structure were performed using OLEX. The networks is constructed
of two distinct 4-connected nodes (heterogenic network). The nodes have
the same short vertex symbol (4284 ) but different
long one which is 4482828888
for the ligand node and 4487878787
for the silver node.
See also the MolDraw Applet
illustrating the structure.
Programming
By the time I came to Nottingham, I had a wide range of experience in computer programming. I started programming when I was fifteen and computer became my best friends since I touched the keyboard for the first time. Since that time I have produced plenty of useless programs and applications, only to gain experience and learning. Programming for Windows® came to me when the third version of Borland® C++ Builder came out. It was not new for the world, but for me it was challenging and absorbing. That time I was using programming as a tool for solving simple tasks for my academic studies. The real challenges appeared only when I started working as a free-lance software tester and as a programmer later. I sold my first software for $50 only!!!!; the next one was sold for $400 and allowed me to get a temporary position which I kept to get some extra income until I came to Nottingham.
I came to Nottingham in the end of January of 2001 with a purpose to do PhD in chemistry under the supervision of Dr Neil R. Champness and Prof Martin Schröder. I spent a few months to get an appropriate skills English. And soon after I passed an English exam I started working on my project.
OLEX: After a discussion with my supervisors I realised that my old crystallographic project, which I had been developing in Russia could have a great future. The main idea was to create new software which would help to analyse extended chemical structures. I had to start writing the software from the beginning as my old project had a very bad structure. But surely I managed to copy and paste some of the hidden bugs, the majority of which apparently were fixed, but wasted a lot of my time. When the graphical software was nearly written, about a year had gone. I did not know how to name the software, but remembered the words of my former QA manager - "the software name determines its future". I had asked the question around our research group and Neil Oxtoby came out with "OLEX" and so it is now. Topological analysis was not very difficult to implement, but efficient algorithms were developed only recently.
LCELLS: At some point during my PhD, Dr A.J. Blake suggested me an idea to create LCELLS, which surely would be very useful tool for crystallographic needs. The basic core of the software was created very quick, within a few days. However some other features such as the cell reduction and processing of ZIP files slowed the process down. The processing of ZIP archives was one of the main problems because the libraries accessible via internet were full of bugs or did not work at all. To solve the problem I decided to use old command prompt based PKWARE® PKZIP compressor, which makes the software dependent on an external program, but at least it works!. When I finished the development, we decided to publish the paper to let people know about the software. The next idea in the development of LCELLS is the introduction of substructure search. A debug version of the new version is developed, and will be released shortly.
References
1. Blake, A.J., et al., Multi-modal bridging ligands; effect of ligand
functionality, anion and crystallisation solvent in silver(I) co-ordination
polymers. J. Chem. Soc., Dalton Trans., 2000: p. 3811-3819.
2. Blake, A.J., et al., Synthesis of a chiral adamantoid network - the role of
solvent in the construction of new coordination networks with silver (I).
Chemical Communications, 2000: p. 665-666.
3. Dolomanov, O.V., et al., A novel synthetic strategy for hexanuclear
supramolecular architectures. Chem. Commun., 2003: p. 682-683.
4. Dolomanov, O.V., et al., A design strategy for four-connected coordination
frameworks. Chem. Commun., 2004: in press.