Quartz (SiO2)
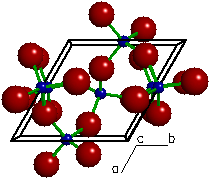
The crystal structure of
Quartz (SiO2)

A "ball and stick" view of the crystal
structure of quartz viewed parallel to the c-axis direction of
the unit cell. The large red spheres represent oxygen atoms, the small blue spheres
represent silicon atoms, the green
rods represent bonds between two
atoms and the black box represents the outline
of the unit cell. The atomic configuration formed here
between a central silicon atom and its four neighboring (or
coordinated) oxygen atoms is called a silicate
tetrahedron.
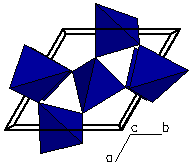 A "polygon" view. The polygon is
constructed by enclosing the central silicon atom (or cation) in
a shell formed by using the positions of the coordinating oxygen
atoms as its' corners. Here we can better see how the atomic
arrangement represents a polygon called a tetrahedron. These
tetrahedra are linked at the corners to form a framework silicate.
A "polygon" view. The polygon is
constructed by enclosing the central silicon atom (or cation) in
a shell formed by using the positions of the coordinating oxygen
atoms as its' corners. Here we can better see how the atomic
arrangement represents a polygon called a tetrahedron. These
tetrahedra are linked at the corners to form a framework silicate.
 A "thermal ellipsoid" view. A thermal
ellipsoid represents the surface enclosed by a trivariate-normal
distribution function that describes the atom's time-averaged
position as it undergoes thermal displacements.
A "thermal ellipsoid" view. A thermal
ellipsoid represents the surface enclosed by a trivariate-normal
distribution function that describes the atom's time-averaged
position as it undergoes thermal displacements.
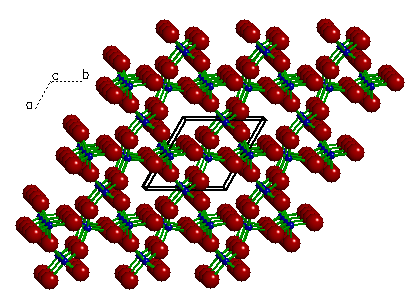 A view of quartz showing the translational symmetry
of the crystal structure. Here we see twenty seven equivalent
unit cells formed by duplicating (or translating) a single unit
cell (outlined). The actual crystal contains millions of these
transitionally equivalent unit cells.
A view of quartz showing the translational symmetry
of the crystal structure. Here we see twenty seven equivalent
unit cells formed by duplicating (or translating) a single unit
cell (outlined). The actual crystal contains millions of these
transitionally equivalent unit cells.
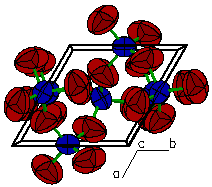
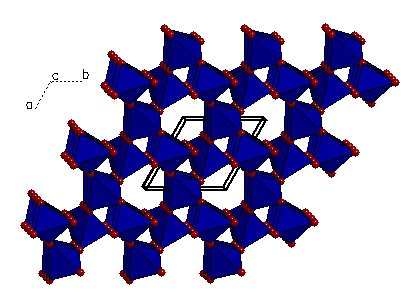 A "polygon" view showing the oxygen atoms
at the corners of each tetrahedron.
A "polygon" view showing the oxygen atoms
at the corners of each tetrahedron.
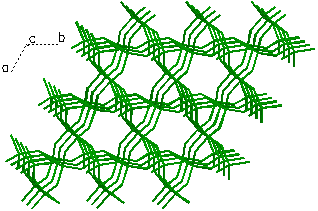 A view showing only Si-O bonds within the quartz
crystal structure. Here a bond is drawn between a silicon atom
(cation) and any oxygen atoms (anions) that are within a 2Å
radius.
A view showing only Si-O bonds within the quartz
crystal structure. Here a bond is drawn between a silicon atom
(cation) and any oxygen atoms (anions) that are within a 2Å
radius.
The above images of the quartz structure were generated using the program Xtaldraw. They are meant to serve as an introduction to the quartz crystal structure as well as to illustrate some of the program features.
Xtaldraw | Quartz | Left/Right handed quartz | Silicate Structures | Garnet Sample data file
![]() June 26, 1996 -
© 1997, Kurt L.
Bartelmehs
June 26, 1996 -
© 1997, Kurt L.
Bartelmehs