 |
 |
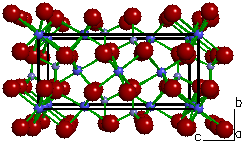
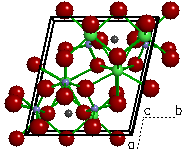
| Figure 1a. The
crystal structure of olivine ((Fe,Mg)2SiO4).
|
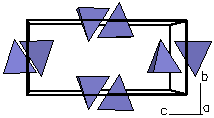
Figure 1b. Looking
only at a polygon view of the silicate tetrahedra, we can
see each is isolated from the other. Because the
structure possesses isolated silicate tetrahedra, olivine
is called an nesosilicate. The
term is derived from the Greek word (nesogaean) that
means "island". |
 |
 |
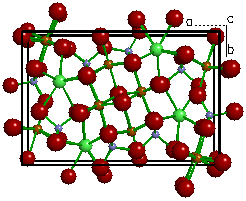
| Figure 2a. The
crystal structure of Ilvaite (CaFe3Si2O8(OH)). |
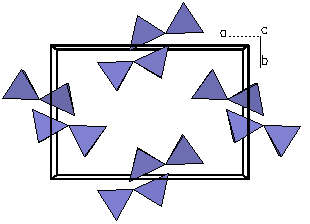
Figure 2b. Looking
only at a polygon view of the silicate tetrahedra, we can
see each tetrahedra is linked at a corner to form pairs.
Because the structure possesses double island silicate
tetrahedra, ilvaite is called an sorosilicate.
The term is derived from a Greek word that means
"group". |
 |
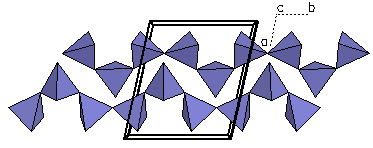 |
| Figure 3a. The
crystal structure of Pectolite (Ca2NaH(SiO3)3). |
Figure 3b. Looking only at a
polygon view of the silicate tetrahedra, we can see each
tetrahedra is linked to two others at the corners to form
single chains. Because the structure possesses parallel
single chains of silicate tetrahedra, pectolite is called
an inosilicate (single chain).
This type of structure is represented by the pyroxenes.
The term is derived from a Greek word that means
"chain". |
 |
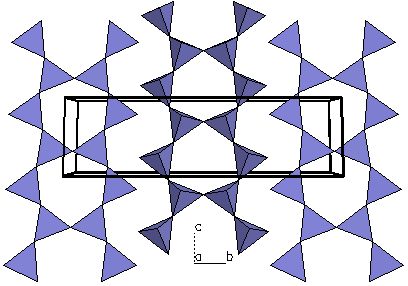 |
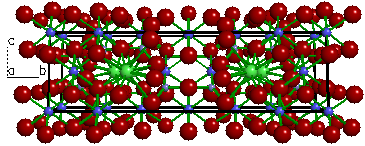
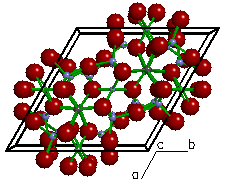
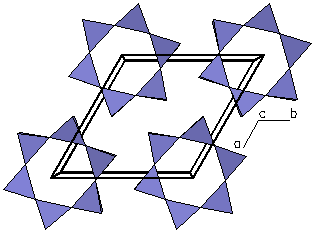
| Figure 4a. The
crystal structure of Tremolite (Ca2Mg5Si8O22(OH)2). |
Figure 4b. Looking only at a
polygon view of the silicate tetrahedra, we can see each
tetrahedra is linked at the corners to form double
chains. Because the structure possesses parallel double
chains of silicate tetrahedra, tremolite is called an inosilicate
(double chain). This type of
structure is represented by the amphiboles. |
 |
 |
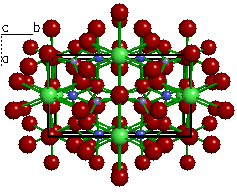
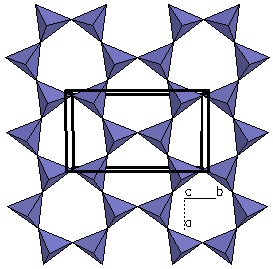
| Figure 5a. The
crystal structure of Beryl (Be3Al2(Si6O18)). |
Figure 5b. Looking only at a
polygon view of the silicate tetrahedra, we can see each
tetrahedra is linked at the corners to form rings.
Because the structure possesses isolated rings of
silicate tetrahedra, beryl is called an cyclosilicate.
The term is derived from a Greek word that means
"ring". |
 |
 |
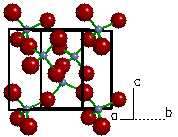
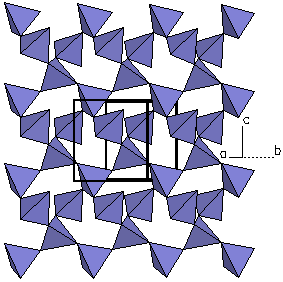
| Figure 6a. The
crystal structure of Biotite (K(Mg,Fe)3(AlSi3O10)(OH)2). |
Figure 6b. Looking only at a
polygon view of the silicate tetrahedra, we can see each
tetrahedra is linked at three corners to form a sheet.
Because the structure possesses parallel sheets of
silicate tetrahedra, biotite is called an phyllosilicate.
This type of structure is represented by the micas.
The term is derived from a Greek word that means
"sheet". |
 |
 |
| Figure 7a. The
crystal structure of Quartz (SiO2). |
Figure 7b. Looking only at a
polygon view of the silicate tetrahedra, we can see that
every tetrahedra is linked at each corner to form a
framework. Because the structure possesses a
three-dimensional framework of silicate tetrahedra,
quartz is called an framework silicate. |
![]() July 16, 1996 -
© 1997 Kurt L.
Bartelmehs
July 16, 1996 -
© 1997 Kurt L.
Bartelmehs