

Research Programs
A wide variety of crystallographic research is performed in the Crystallography Laboratory
at Virgina Tech. Here is a brief overview of current research projects and recent
results. Also, have a look at the links from the personal pages (follow the people link at left).
Lists of recent publications from the laboratory are available as pdf files:
2004 Publications
2005 Publications
Frameworks
Nancy Ross and Ross Angel have been funded by NSF grants EAR-0105864 and EAR-0408460
to determine the role that bond compression plays in the compression of structures that can be
described as polyhedral frameworks. It has often been assumed that such structures (feldspars, zeolites, perovskites)
compress solely by the tilting of rigid atomic groups (or polyhedra), without compressing the metal-oxygen bonds of the framework.
With the support of these two grants we have been able to develop the experimental techniques to measure extremely
small bond compressions in structures at high pressures, and to show that in some structures bond compression in the
framework, although small, determines the high pressure behavior of the mineral. These small changes in structure under
pressure will have a large effect on cation partitioning between phases within the Earth, will influence the retention
and diffusion rates of non-bonded species such as the rare gases that are important for isotope geochemistry, and will
determine the elastic properties of these minerals.
Feldpsars and coesite
Both coesite and feldspars display complex variation of their volume under compression, but this can be explained in terms of the response of frameworks being that of essentially rigid tetrahedra. The compression of some Si-O bonds in coesite serves only to modify the rigid-unit behaviour of the framework. The following links provide reprints of the publications describing these results:
Plagioclase Eos
Coesite Eos
This paper reports the determination of the full elasticity tensor of albite, the first time this has been done for a triclinic mineral.
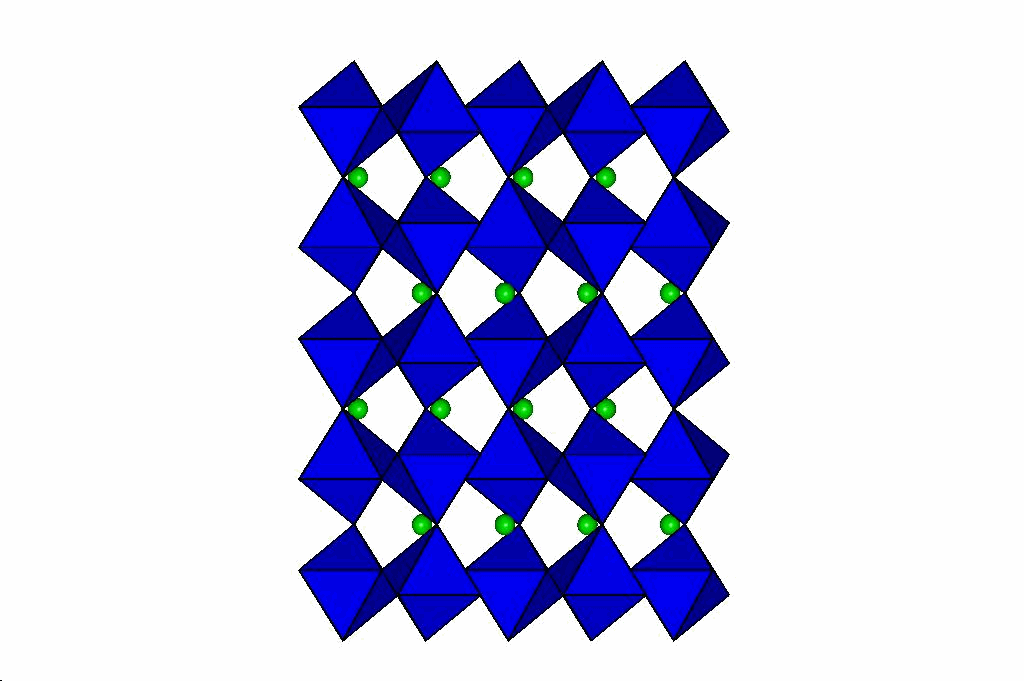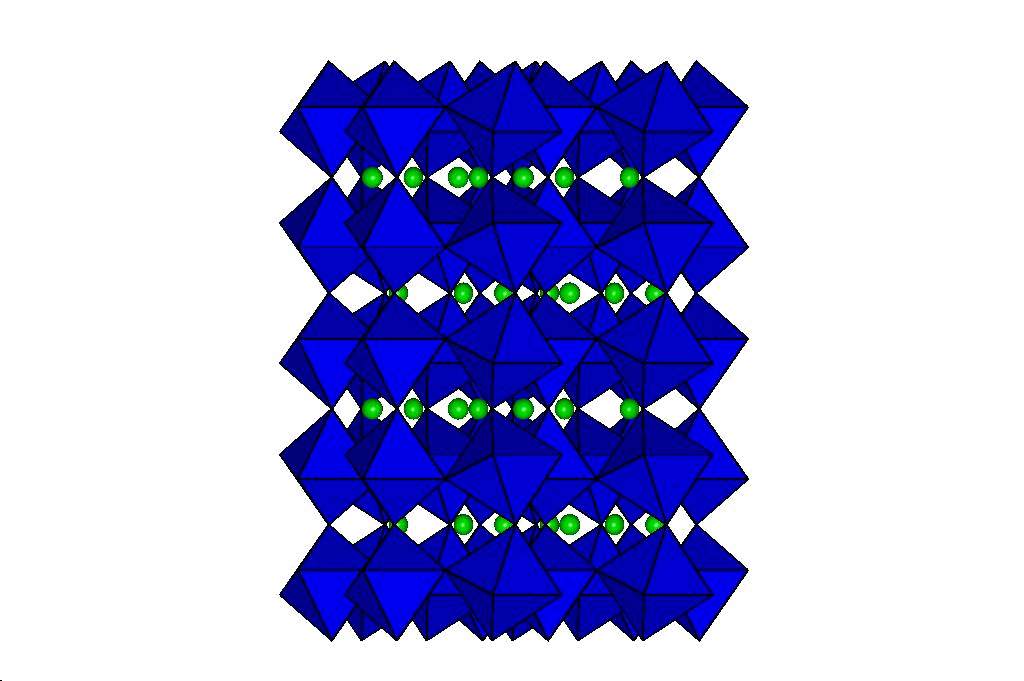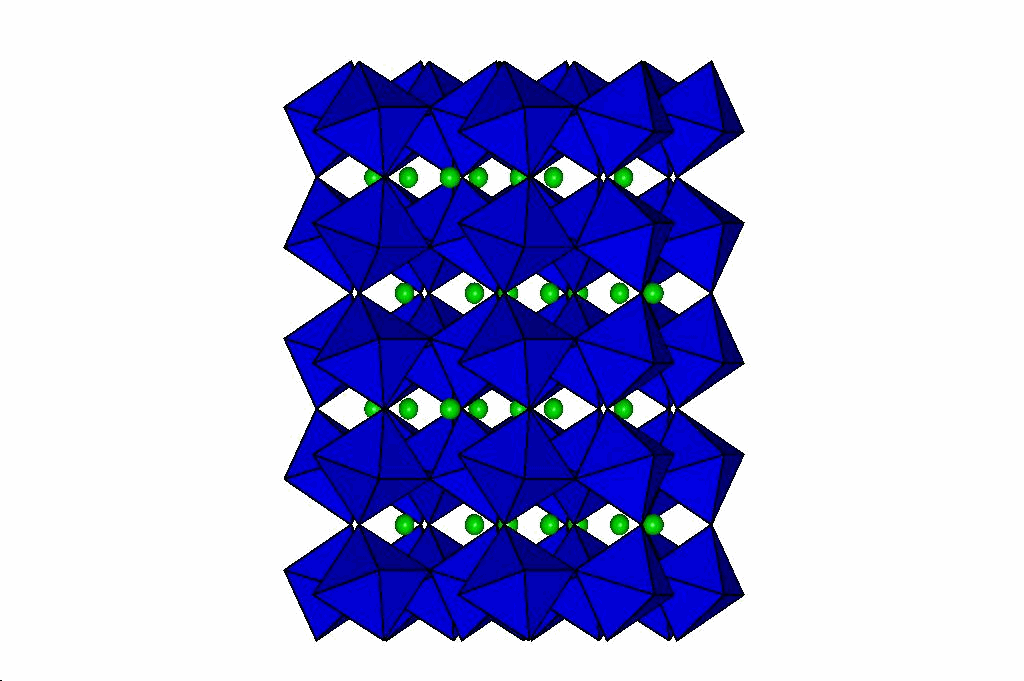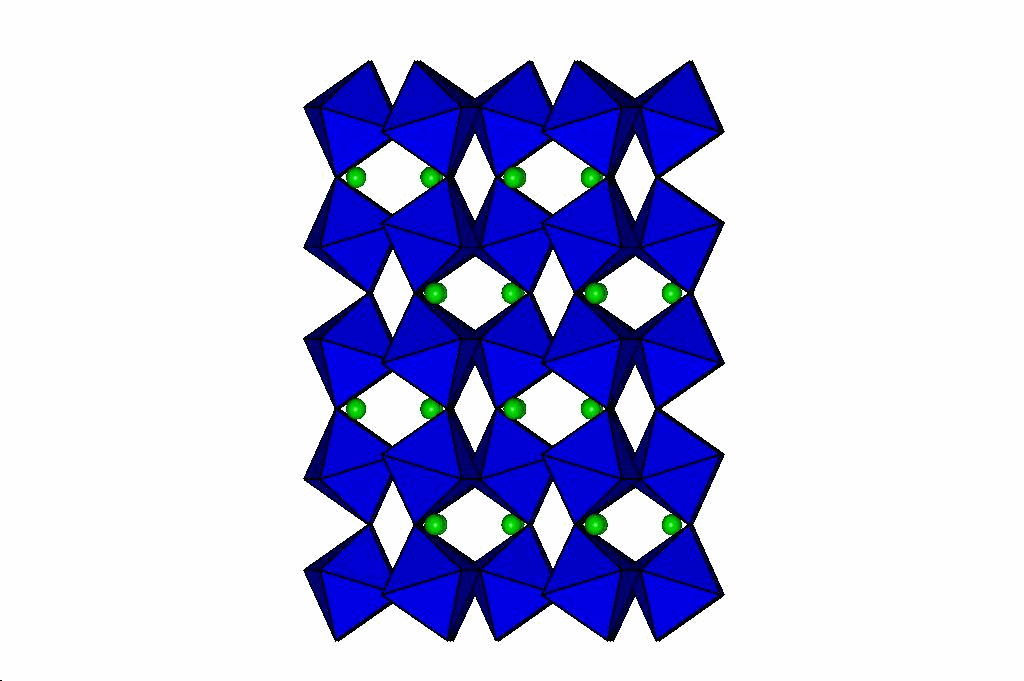
Perovskites
Through a series of high-pressure structural studies of various perovskites we have determined that bond compression
in the octahedra determines the evolution of the perovskite at high pressures. If the octahedra are less compressible
than the A-cation site, then the structure becomes more distorted at high pressure. Conversely, if the octahedra are
more compressible than the A cation site, then pressure reduces the distortion of the perovskite, and can even lead to phase transitions
to higher-symmetry structures. From these results we have developed a model that will predict the behaviour of
perovskites at high pressure which is described in this reprint. Our latest
results on silicate perovskites relevent to the Earth's lower mantle are in this reprint.
For further information about our current experiments on perovskites, please contact
Dr. Jing Zhao.
Feldpsars and coesite
Both coesite and feldspars display complex variation of their volume under compression, but this can be explained in terms of the response of frameworks being that of essentially rigid tetrahedra. The compression of some Si-O bonds in coesite serves only to modify the rigid-unit behaviour of the framework. The following links provide reprints of the publications describing these results:
Plagioclase Eos
Coesite Eos
This paper reports the determination of the full elasticity tensor of albite, the first time this has been done for a triclinic mineral.

Phase Transitions
Ross Angel has been studying phase transitions at high pressures for many years. While the physics of structural phase
transitions, even in complex systems, are now well-understood at high and low temperatures, our exploration of
the same transitions at high pressure is still in its infancy. Of particular interest are systems that behave in
unusual ways, for example;
Clinopyroxenes undergo a sequence of transitions with space group changes C2/c -> P21/c -> C2/c on increasing
pressure. Some of these transitions can be seen in the optical microscope.

But we are interested in the factors that determine the relative stabilities of the three phases, and also whether it is possible to drive a direct phase transition from C2/c to C2/c, which would be of fundamental importance as phase changes without symmetry change are extremely rare.
Lead phosphate is an improper ferroelastic, which undergoes a transition from monoclinic to trigonal symmetry on increasing temperature or pressure. Whereas the high-symmetry structure is dynamically disordered at high temperatures our recent high-pressure neutron powder diffraction study that it is statically disordered at high pressures. There are two papers describing these results:
Pb-phosphate paper 1
Pb-phosphate paper 2 This paper includes some diffuse scattering measurements, the results of which can also be viewed in more detail here.
The same general behaviour appears to occur in anorthite feldspar (Angel, 1988: American Mineralogist 73:1114-1119). By contrast, the high-pressure, high-symmetry phase of titanite is statically ordered compared to its disordered high-temperature phase (Angel et al. 1999: Phase Transitions, 68:533-543).

But we are interested in the factors that determine the relative stabilities of the three phases, and also whether it is possible to drive a direct phase transition from C2/c to C2/c, which would be of fundamental importance as phase changes without symmetry change are extremely rare.
Lead phosphate is an improper ferroelastic, which undergoes a transition from monoclinic to trigonal symmetry on increasing temperature or pressure. Whereas the high-symmetry structure is dynamically disordered at high temperatures our recent high-pressure neutron powder diffraction study that it is statically disordered at high pressures. There are two papers describing these results:
Pb-phosphate paper 1
Pb-phosphate paper 2 This paper includes some diffuse scattering measurements, the results of which can also be viewed in more detail here.
The same general behaviour appears to occur in anorthite feldspar (Angel, 1988: American Mineralogist 73:1114-1119). By contrast, the high-pressure, high-symmetry phase of titanite is statically ordered compared to its disordered high-temperature phase (Angel et al. 1999: Phase Transitions, 68:533-543).
Molecules at High Pressure
Most of the high-pressure crystallographic studies have been performed on inorganic materials and especially
minerals. Ross Angel, Carla Slebodnick and Maciej Bujak, in collaboration with members of the Chemistry Department, are now
starting projects in which we use pressure to probe the balance between the strong intra-molecular
forces (bonds) and the weaker inter-molecular forces within molecular solids.
As we apply pressure to molecular solids the molecules will
move together and the inter-molecular repulsive forces will increase. As a result we can suppress or halt dynamic
behaviour of molecules, study phase changes and induce new properties in substances which can later be mimicked at
atmospheric pressure by rational design and synthesis. As an example, read about the phase transitions and bonding changes
we have found in a novel organic-inorganic hybrid structure at high pressure. With Prof. Brian Hanson
we are probing metal-carbonyl compounds to determine whether the widely-held belief that their conformations are
a product of steric interactions - if they are then we expect to induce phase transitions from one conformation to another
by the application of pressure.