Introduction to
The Unit Cell
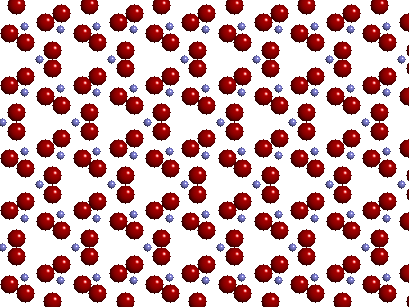
The image above depicts the two-dimensional projection of the crystal structure for the mineral quartz (Dimensions: ~ 25Å × 20Å) . The blue spheres represent Si (silicon) atoms and the red spheres represent O (oxygen) atoms. What do you observe in the image above? Do see a repeating pattern like you might see in some kind of wallpaper? If so, what is the pattern?
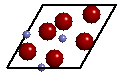 If you answered yes, then you may have
chosen the pattern of atoms outlined in the image to the right. This portion of the
crystal structure is called the unit cell (solid outline). The unit cell
is that unique part of the crystal structure such that when translated along
parallel lines, generates the entire crystal structure. We can see that the unit cell of
quartz has three Si atoms and six O atoms in its unit cell in a ratio of 3:6. The chemical
formula of quartz is therefore Si3O6 but can
be reduced (by dividing the number of each atom by 3) to SiO2. The image below
shows how the unit cell (right) can be translated from left to right to generate the
crystal structure of quartz (two-dimensional) that is shown in the top
image.
If you answered yes, then you may have
chosen the pattern of atoms outlined in the image to the right. This portion of the
crystal structure is called the unit cell (solid outline). The unit cell
is that unique part of the crystal structure such that when translated along
parallel lines, generates the entire crystal structure. We can see that the unit cell of
quartz has three Si atoms and six O atoms in its unit cell in a ratio of 3:6. The chemical
formula of quartz is therefore Si3O6 but can
be reduced (by dividing the number of each atom by 3) to SiO2. The image below
shows how the unit cell (right) can be translated from left to right to generate the
crystal structure of quartz (two-dimensional) that is shown in the top
image.
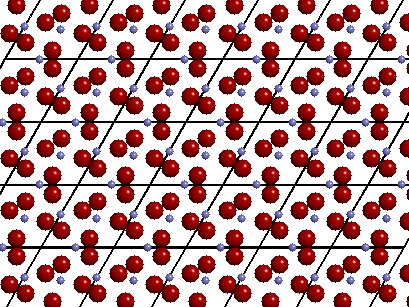
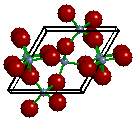 However,
the traditional drawing of the "unit cell" is slightly different. As you can see
in the image above, every Si is surrounded by four nearest
neighbor O atoms. These nearby O atoms (anions) are said to be bonded (or
coordinated) to the central Si atom (cation). A bond is
typically represented by drawing a rod (or stick) between each bonded atom. The image on
the right shows the traditional "ball and stick" view of the unit cell of
quartz. To complete the coordination sphere of each Si (in this case, the four O atoms
within 2Å of a Si) and O, some O and Si atoms in each adjacent unit cells are drawn, i.e.
atoms are drawn outside of the box.
However,
the traditional drawing of the "unit cell" is slightly different. As you can see
in the image above, every Si is surrounded by four nearest
neighbor O atoms. These nearby O atoms (anions) are said to be bonded (or
coordinated) to the central Si atom (cation). A bond is
typically represented by drawing a rod (or stick) between each bonded atom. The image on
the right shows the traditional "ball and stick" view of the unit cell of
quartz. To complete the coordination sphere of each Si (in this case, the four O atoms
within 2Å of a Si) and O, some O and Si atoms in each adjacent unit cells are drawn, i.e.
atoms are drawn outside of the box.
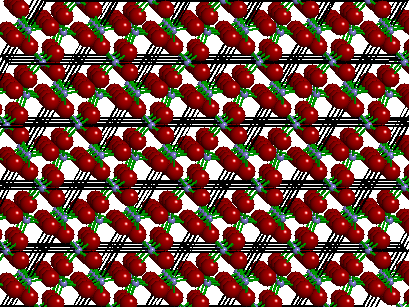
The image below depicts the three-dimensional crystal structure of the mineral quartz showing the bonding and outlines of each unit cell. Observe how a crystal structure (in this case quartz) can be generated by simply translating or repeating a single unit cell along parallel lines in three dimensions. Though the "real" crystal structure contains millions of unit cells, a crystal chemical study requires that we only need to study the "unique" atoms that make up a single unit cell.
Xtaldraw | Quartz | Left/Right handed quartz | Silicate
Structures | Garnet Sample data file
Unit Cell
![]() April 8, 1997 - © 1997, Kurt L. Bartelmehs
April 8, 1997 - © 1997, Kurt L. Bartelmehs